A few comments about our two electrolysis experiments:
Here's what I want you to understand-->>>
First of all, doing the experiment gives you hands-on experience in observing chemical reactions. It is important to follow directions carefully, including what's in the diagram, so that you get your setup just right. Then it is important to be patient, not everything is an explosion, or has an immediately visible reaction. Sometimes no reaction is what is happening, and sometimes a slow reaction is what you have to observe --patience and attention to detail!
- In these experiments, the main idea is that transferring electrons changes the chemical and physical properties of that atom, DRAMATICALLY!
- In the first lab, the chloride ion [Cl-] lost an electron, combined with another Cl-, and bubbled up as chlorine gas. Likewise, the H+ atoms gained an electron, combined to form H2 and bubbled up as a gas.
- In yesterday's demonstration, the copper (II) ion, which was dissolved in the blue solution, gained two electrons and became the regular metallic copper that we are used to seeing on a penny. We did not observe it, but the sulfate ion [SO42- ] lost two electrons, combined with hydrogen, and became sulfuric acid.
- It took energy (from the battery) to make these reactions happen.
- A chemical reactions is when compounds break apart and recombine to form different combinations of atoms.
- I also wanted you to see examples of chemical equations look like, just to get used to them:
- 2H+ + 2e-
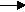 H2 (hydrogen gas forms at the (-)cathode).
H2 (hydrogen gas forms at the (-)cathode).
2Cl- - 2e-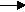 Cl2 (chlorine gas forms at the (+)anode).
Cl2 (chlorine gas forms at the (+)anode). - 2NaCl(aq) + 2H2O(l)
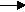 2Na+(aq) + 2OH-(aq) + Cl2(g)+ H2(g)
2Na+(aq) + 2OH-(aq) + Cl2(g)+ H2(g) - Matter is not created or destroyed-- The Law of the Conservation of Matter-- means that if we consider matter at the atomic level, the small particles are not created or destroyed. We can trace these atoms and molecules through a system. The same atoms present at the beginning are there at the end, just in different combinations.
NOMENCLATURE FOR COVALENT BONDING
Worksheet # 3 from this
http://www.npsd.k12.nj.us/cms/lib04/NJ01001216/Centricity/Domain/474/Ch%209%20WS%20Ionic%20Tranisition%20Covalent%20Nomenclature%20Packet.pdf
and the answers:
For INSTRUCTIONS, SEE YOUR TEXTBOOK, p. 592-593 and/or watch this:
Memorize the PREFIXES
optional, but interesting, no notes necessary.
