same thing...leave a comment after you've watched the video.
I really enjoyed this video a lot.
When Chemicals Meet Water
Really fine job on the show yesterday! Bravo!
Friday, May 29, 2015
Wednesday, May 27, 2015
Tomorrow, the Show
Homework is simply to be prepared for tomorrow's science show. Please be on time, 9:10, so we can set up the room and ourselves and work out any problems. Show starts at 10. Call me today if you have questions or supply needs. We will just do our best, enjoy ourselves, and let the show go on!
Connor, I am supposed to bring you two things, could you remind me what they are? the thiosulfate and something else?
Ian, you still have time to come up with something, how about what we did with the steam and the crunching pop can? That would be impressive! http://www.stevespanglerscience.com/lab/experiments/incredible-can-crusher
Connor, I am supposed to bring you two things, could you remind me what they are? the thiosulfate and something else?
Ian, you still have time to come up with something, how about what we did with the steam and the crunching pop can? That would be impressive! http://www.stevespanglerscience.com/lab/experiments/incredible-can-crusher
Monday, May 18, 2015
AVOID WEEKEND HOMEWORK...
WATCH TWO VIDEOS...
ONE ON TUESDAY,
AND ONE ON WEDNESDAY
or you can do it the regular way,
one before Thursday
and one before the next class (next Tuesday)
Same routine, watch video + leave a comment
watch the other video + leave a comment for that.
#1: Unit 6: Quantifying Chemical Reactions—Stoichiometry and Moles
#2 Unit 7: The Energy in Chemical Reactions—Thermodynamics and Enthalpy
WATCH TWO VIDEOS...
ONE ON TUESDAY,
AND ONE ON WEDNESDAY
or you can do it the regular way,
one before Thursday
and one before the next class (next Tuesday)
Same routine, watch video + leave a comment
watch the other video + leave a comment for that.
#1: Unit 6: Quantifying Chemical Reactions—Stoichiometry and Moles
#2 Unit 7: The Energy in Chemical Reactions—Thermodynamics and Enthalpy
Friday, May 15, 2015
Today' homework (Friday) is another review/preview video. As before, leave a comment describing a section of the video in the comments below:
Unit 5: Making Molecules—Lewis Structures and Molecular Geometries
And here's my comment: At around 20 minutes in the video, the MIT chemist talks about what made her want to switch from becoming a doctor to becoming a chemist, and her words were these:


note: last period I received comments from Laura, Niles, Abigail, Connor and Zach. Good job, to them, but I expect ALL of you to watch and make a significant comment.
Monday we will practice the chem show. Bring in any extra items you will need.
Unit 5: Making Molecules—Lewis Structures and Molecular Geometries
And here's my comment: At around 20 minutes in the video, the MIT chemist talks about what made her want to switch from becoming a doctor to becoming a chemist, and her words were these:
So, when I first studied penicillin, I was amazed, I said ‘Wow, like, so this, thinking about the structure right here is how we can actually understand how it works in the body. And this is the point for me where I started that switchover from thinking I want to help people by becoming a doctor to I want help people by studying and understanding how things work on a molecular level.What stood out to me is her choosing a career based on how "I want to help people," not just what I like doing or what makes a lot of money or brings me fame, but how I can help...How great is that!
note: last period I received comments from Laura, Niles, Abigail, Connor and Zach. Good job, to them, but I expect ALL of you to watch and make a significant comment.
Monday we will practice the chem show. Bring in any extra items you will need.
Tuesday, May 12, 2015
Here's the video; after you watch, jot down a little bit about a section from the video, and leave it in the comments below, along with your REAL NAME:) Unit 4: Organizing Atoms and Electrons—The Periodic Table


Friday, May 8, 2015
Homework Today (Friday) is to watch another of these excellent REview/PREview videos; this one is on the electron and the atomic model. One thing I did not cover in class is the relationship between electrons and light, which would be particularly useful to understand Mabel and Abigail's chemagic demonstrations. Like last period, so that I know you have done your homework, write one sentence stating an important point from the video, and leave that, along with your name, in the COMMENTS below. I noticed only 4 people left comments last homework.
Unit 3: Atoms and Light—Exploring Atomic and Electronic Structure
Unit 3: Atoms and Light—Exploring Atomic and Electronic Structure
Tuesday, May 5, 2015
For homework this period, we will continue the chemistry video series we began last week. This series of videos is a REview/PREview; it recaps the major points we have studied (the first 15 minutes) and then it goes beyond that in interesting ways. So the beginning of each video should be familiar, and the end might be a little complicated, but worthwhile.
So, lesson 2, Unit 2: The Behavior of Atoms—Phases of Matter and the Properties of Gases
When you have finished watching the video, please leave a comment stating one thing mentioned in the video, along with your name.
#2. Work on the dialogue (what you will say) to go along with your chemagic demonstration.
Please be at the Copperman's by 9:15 on Thursday. HAVE YOU LEFT A COMMENT?
So, lesson 2, Unit 2: The Behavior of Atoms—Phases of Matter and the Properties of Gases
When you have finished watching the video, please leave a comment stating one thing mentioned in the video, along with your name.
#2. Work on the dialogue (what you will say) to go along with your chemagic demonstration.
Please be at the Copperman's by 9:15 on Thursday. HAVE YOU LEFT A COMMENT?
Friday, May 1, 2015
Three things:.
2. The CheMagic Show -- On Monday we will work on this in small groups, I will bring the supplies, as much as I know of, and you MUST bring a printout of directions for your act, and any props you need. Class still starts at 9:10, even if we start with 4square. Don't be late.
3. ...and the homework, a video review of first principles: http://www.learner.org/courses/chemistry/video/vidbyunit_1.html
30 minutes
4. and also review-read chapter 25, sections 1&2, and read section 3 (no notes, but highlight/underline what you think you might want to remember for a quiz)
2. The CheMagic Show -- On Monday we will work on this in small groups, I will bring the supplies, as much as I know of, and you MUST bring a printout of directions for your act, and any props you need. Class still starts at 9:10, even if we start with 4square. Don't be late.
3. ...and the homework, a video review of first principles: http://www.learner.org/courses/chemistry/video/vidbyunit_1.html
30 minutes
4. and also review-read chapter 25, sections 1&2, and read section 3 (no notes, but highlight/underline what you think you might want to remember for a quiz)
Tuesday, April 28, 2015
The Charge of the Proton! It's ENERGY, it's ACTION, it's Something Happening!
So...my computer is currently uploading a video message from me, but it is going slow, and I don't know how it will turn out...wait>>>IT WORKED! A new era dawns! Imagine the possibilities; I can talk to you about yesterday's experiment or try again to explain a difficult concept! WOWsers.
Today's Homework:
1. pHet simulation: https://phet.colorado.edu/sims/html/acid-base-solutions/latest/acid-base-solutions_en.html THOROUGHLY explore this simulation, using all the Solutions and Tools, first in the Introduction and then in My Solution, - figure out how the sim works and then learn from it.
- When you do the My Solutions page >>> MAKE A TABLE, like this: https://drive.google.com/file/d/0B07s3-bcUr7tYS1Lcl9jdkFFaHM/view?usp=sharing
- Change the acid/base concentrations and strengths
- dip the pH meter in the water, dip the litmus paper, use the light bulb to test conductivity
- notice the chemical equation, what does HA mean, etc./ (H is H+ and A represents acid) MOH? M represents the metal ion and OH is OH-. Notice that a strong acid uses a one-direction arrow and a weak acid uses arrows going in both directions. What does this represent? Notice the differences in the diagram as you switch from strong to weak acid.
2. A really excellent 30 minute video deserving the full concentration (and strength) of your attention:
- Acids and Bases: The Voyage of the Proton Don't forget to pound it into your brain: H+ is a proton, so pH is the Power of the Proton, it has a charge, and a charge is like a battle cry, Charge! It's energy, it's action, it's Something Happening!
Friday, April 24, 2015
More Acids
1. From our in-class experiment: as I explained in class, summarize your conclusions from each section. I EXPLAINED IT IN CLASS, Do it according to the model I wrote on the board.
2. Read textbook, chapter 25, section 2: Strength of Acids and Bases. Take good notes. List each section and sub-section, write 2-4 bullet points which tell the important points and what must be remembered from that section, including vocabulary and reading checks. Copy Figures 7, 8, 9 and 11. If you don't know what a word means, figure it out, and write it in the book before you go on. For instance, "ionizes" and "dissociates"--do you know what it means?
3. Brainpop: Brainpop >>> pH scale
username: pcshome password: ilearnathome
Watch movie, do quiz, read FYI, fill out the activity page OR do one of the games in Game-UP.
A few of you have not yet turned in a description of your Chemistry Magic Show demonstration, and I need to talk with some of you about whether yours will work. Can't remember who is doing the mentos experiment--you need to find something that can be done indoors. ALL OF YOU need a complete printout of your experiment and have it with you in class. If you need help choosing something, tell me.
Tuesday, April 21, 2015
Properties of Acids and Bases
- IF YOU MISSED MONDAY'S CLASS: WATCH THIS VIDEO AND TAKE NOTES:
BrainPOP: username: pcshome password: ilearnathome
https://www.brainpop.com/science/matterandchemistry/acidsandbases/
watch movie, take quiz, read all sections of FYI (click the round icons at the top of the page)
Friday, April 10, 2015
Spring break, no homework. EXCEPT...
I will be calling you individually to discuss your ChemMagic proposals. If you did not turn it in yet, do this right now! I also need everyone to send me the link to your reaction in the COMMENTs BELOW. One more thing to consider is that you need to write the chemical equation of your proposed reaction. I will be reviewing each of your proposals to see that they fit the criteria and do not overlap. So there will probably be some plan changes, just sayin'.
Those of you who did not turn in the completed Chemical Reactions Lab report, do so NOW.
If you've lost the original handout, here it is: http://www.kwanga.net/chemnotes/chem-rxns-lab.pdf
I will be calling you individually to discuss your ChemMagic proposals. If you did not turn it in yet, do this right now! I also need everyone to send me the link to your reaction in the COMMENTs BELOW. One more thing to consider is that you need to write the chemical equation of your proposed reaction. I will be reviewing each of your proposals to see that they fit the criteria and do not overlap. So there will probably be some plan changes, just sayin'.
Those of you who did not turn in the completed Chemical Reactions Lab report, do so NOW.
If you've lost the original handout, here it is: http://www.kwanga.net/chemnotes/chem-rxns-lab.pdf
Tuesday, April 7, 2015
More Magic
One of you ambitious students requested this experiment, which sadly I couldn't approve-- making salt from sodium and chlorine; it would've been great: check out this video from Theo Gray's Mad Science--->>> http://graysci.com/chapter-one/making-salt-the-hard-way/
Plus there are lots of experiments from his site (look under the chapter tabs on the banner). http://graysci.com/ Mostly too dangerous.
Steve Spangler has lots of ideas and demonstration videos on his catalog, and the advantage to looking here is that these are reasonably safe, and the supplies are available. http://www.stevespanglerscience.com/lab/experiments/category/chemistry
YouTube has tons of stuff, try searching for chemistry magic tricks, Crazy Russian Hacker, you can also do a demonstration that I have done in class.
email or text me the link, if you find one you think is possible, or find several, and I'll get back to you.
Plus there are lots of experiments from his site (look under the chapter tabs on the banner). http://graysci.com/ Mostly too dangerous.
Steve Spangler has lots of ideas and demonstration videos on his catalog, and the advantage to looking here is that these are reasonably safe, and the supplies are available. http://www.stevespanglerscience.com/lab/experiments/category/chemistry
YouTube has tons of stuff, try searching for chemistry magic tricks, Crazy Russian Hacker, you can also do a demonstration that I have done in class.
email or text me the link, if you find one you think is possible, or find several, and I'll get back to you.
Monday, March 30, 2015
The Magic
Thank you for your attention during class; it was a fun day. This Thursday we will devote our science and art time to doing the Easter garden, so bring the basket or terra cotta saucer, or if you are unable to find something, text me (415 858 4191). Also, $5 for materials would be appreciated.

Some of you have asked when we will do the chemistry magic show; not till after spring break, perhaps the 23rd of April, but I want to order supplies by April 10th, so you need to submit your plan and materials request by then. I will order from hometrainingtools.com, so check there to see if what you need is available. I want this presentation to be REALLY WELL PREPARED, with practice,and also safe. Another site with good ideas is stevespanger.com.
Those of you who have not yet turned in the chemical reactions lab, I will be calling your parents--this is NOT OPTIONAL. And it's not a test; if you need help, ask a friend or ask me. If you get to class at 9:10 I will give you my whole attention. If I still don't have it by next Monday, I will ask that you stay and do it during PE time. If this stuff is hard, you need to be asking more questions.
Last of all, here's the new homework, a very long video, more like a movie, really, so get the family together, make popcorn, Chromecast it on your TV (but if that doesn't happen, you still need to watch it on your computer) and enjoy:
Some of you have asked when we will do the chemistry magic show; not till after spring break, perhaps the 23rd of April, but I want to order supplies by April 10th, so you need to submit your plan and materials request by then. I will order from hometrainingtools.com, so check there to see if what you need is available. I want this presentation to be REALLY WELL PREPARED, with practice,and also safe. Another site with good ideas is stevespanger.com.
Those of you who have not yet turned in the chemical reactions lab, I will be calling your parents--this is NOT OPTIONAL. And it's not a test; if you need help, ask a friend or ask me. If you get to class at 9:10 I will give you my whole attention. If I still don't have it by next Monday, I will ask that you stay and do it during PE time. If this stuff is hard, you need to be asking more questions.
Last of all, here's the new homework, a very long video, more like a movie, really, so get the family together, make popcorn, Chromecast it on your TV (but if that doesn't happen, you still need to watch it on your computer) and enjoy:
The Magic of Chemistry (82 minutes)
Lastly, there will be no other new homework till after Easter, so enjoy Good Friday and Resurrection Sunday. God is good!
Friday, March 27, 2015
studying
Those of you in class know the homework, to study in order to fill in the gaps for the quiz.
Look over the past blogs, the set of worksheets, past videos, the quizlet, the textbook, your notes, and come back on Monday ready to nail it.
for fun: (and to look out for, what makes reactions go faster?) watch the videos below.
and keep poking around for the Chemistry Magic demonstrations--email me with what you want to do and what you need.
Also, REMEMBER ABOUT THE EASTER GARDEN DISH OR BASKET -- 2-3 inched deep and about 15" across. Tell your moms now so they can be looking out.
Look over the past blogs, the set of worksheets, past videos, the quizlet, the textbook, your notes, and come back on Monday ready to nail it.
for fun: (and to look out for, what makes reactions go faster?) watch the videos below.
and keep poking around for the Chemistry Magic demonstrations--email me with what you want to do and what you need.
Also, REMEMBER ABOUT THE EASTER GARDEN DISH OR BASKET -- 2-3 inched deep and about 15" across. Tell your moms now so they can be looking out.
Monday, March 23, 2015

Have you done Quizlet before? It's very helpful. I created a vocab list to help you study.
Vocabulary review for chapter 24: http://quizlet.com/76117753/chemical-reactionschapter-24-flash-cards/ try out the different activities on the Header
[Study Flashcards Learn Speller Test Play Scatter Space Race]
Review Chapter 24 and your science journal notes/handouts.
Go over the Study Guide, page 760-761.
Do the Assessment at the end of chapter 24 (page 762-763.
Turn in the Chemical Reactions Lab (16 reactions) on Thursday.
Thank you for your attentiveness in class today; it encourages me more than you can know. COMBUSTION!



 _
_

Friday, March 20, 2015
Some of you left your homework sheet in class: https://drive.google.com/file/d/0B07s3-bcUr7tVVdkYndiTmNhZ1E/view?usp=sharing There are notes and SOME PRACTICE PROBLEMS TO DO ON THE BACK PAGE. We will have a quiz on Monday. Also, turn in your chemical reactions lab on Monday, and make sure that it is neatly done in ink, according to the directions given in class and in previous blogs.
Worksheet to complete: Balancing Combustion Reactions
Quiz will be on identifying the five types of chemical reactions and on the vocabulary from chapter 24, and balancing equations.
Poke around the internet for a chemical reaction demonstration to do. No thermite, but I can order stuff for you from hometrainingtools.com or I might have it in my supply closet. The theme of our "show" will be Chemistry Magic, so pick something with a little dazzle, but still safe. I also have some books with demonstrations/experiments that you can do with household items. We will do this with an audience of moms/dad and siblings.
Worksheet to complete: Balancing Combustion Reactions
Quiz will be on identifying the five types of chemical reactions and on the vocabulary from chapter 24, and balancing equations.
Poke around the internet for a chemical reaction demonstration to do. No thermite, but I can order stuff for you from hometrainingtools.com or I might have it in my supply closet. The theme of our "show" will be Chemistry Magic, so pick something with a little dazzle, but still safe. I also have some books with demonstrations/experiments that you can do with household items. We will do this with an audience of moms/dad and siblings.
Monday, March 16, 2015
1. Re-read Chapter 24, sections 3 & 4: take notes
2. If you have not done the worksheets from previous blogs, do so now:
- http://www.sciencegeek.net/Chemistry/chempdfs/EquationsWorksheet4.pdf
- http://www.sciencegeek.net/Chemistry/chempdfs/EquationsWorksheet5.pdf
- (see previous blog for note about "activity series"
3. To turn in: Write up the set of experiments we did in class in the manner I explained\
- Lab#: description or title
- Observations
- Balanced equation
- If we skipped the lab, omit the observations but do the balanced equation.
- DUE MONDAY, March 23 and it must be legible and written in PEN or typed. NO MECHANICAL PENCILS - but do it now so that you can ask questions on Thursday.
Friday, March 13, 2015
interesting interim
The homework due Monday is the stuff from last blog posting, so scroll down to make sure you have done it all, basically the experiment (writing the equations), the video, and the two worksheets. Monday will be more experiments - single and double displacements (also called replacements).
But here is an interesting article, on the nature side of things from the blog of a remarkable photographer: http://thesmallermajority.com/2015/03/12/mozambique-diary-red-headed-flies/

But here is an interesting article, on the nature side of things from the blog of a remarkable photographer: http://thesmallermajority.com/2015/03/12/mozambique-diary-red-headed-flies/

Monday, March 9, 2015
I plan to skip science class this Thursday and just have a long art class. Please come to art prepared with photos and ideas. Just this once, we will start class at 9:30.
This science homework is to be completed by Monday; DO NOT put it off to the weekend, please.
Finishing parts I and II from the lab... Synthesis and Decomposition experiments (1-7)
In your science journals, go back to each experiment, including the ones we did NOT do, and figure out the balanced equations for each synthesis and decomposition reaction. I will write the answers on the board on Thursday so you can check.
Don't lose your experiment sheets, we will do the rest of the labs next Monday/some of them, anyway.
TAKE NOTES ON VIDEO!!!
This is some extra information you might need for doing Worksheet 4.
Single Displacement Reactions: A + BC -> B + AC
These reactions only happen if metal A is more reactive than metal B.
You can tell which metal (A or B) is more reactive by either doing the experiment, or looking it up on a list called a "reactivity series".
The metals at the top are more reactive than the ones below.

For instance, zinc will replace iron but not magnesium, because zinc is higher on the list than iron, but lower than magnesium. Top guy wins.
Understanding this is not that important at this point, but I know some of you like to be precise just so you know that some reactions are likely to happen, and some are not.
Do these worksheets:
http://www.sciencegeek.net/Chemistry/chempdfs/EquationsWorksheet4.pdf
and http://www.sciencegeek.net/Chemistry/chempdfs/EquationsWorksheet5.pdf
[some of these reactions also won't really happen, as you will see from the answer sheet;
don't worry about trying to predict this with the solubility table, just do the switcharoo >>
AB + CD -> AD + CB and make sure your compounds are metalfirst,thennonmetal.
........................................
Hope you are enjoying the labwork, and putting it together with the worksheets, and starting to "get it."
I appreciate your feedback; we are in the home stretch of chemistry; keep up the good work,
This science homework is to be completed by Monday; DO NOT put it off to the weekend, please.
Finishing parts I and II from the lab... Synthesis and Decomposition experiments (1-7)
In your science journals, go back to each experiment, including the ones we did NOT do, and figure out the balanced equations for each synthesis and decomposition reaction. I will write the answers on the board on Thursday so you can check.
Don't lose your experiment sheets, we will do the rest of the labs next Monday/some of them, anyway.
TAKE NOTES ON VIDEO!!!
This is some extra information you might need for doing Worksheet 4.
Single Displacement Reactions: A + BC -> B + AC
These reactions only happen if metal A is more reactive than metal B.
You can tell which metal (A or B) is more reactive by either doing the experiment, or looking it up on a list called a "reactivity series".
The metals at the top are more reactive than the ones below.

For instance, zinc will replace iron but not magnesium, because zinc is higher on the list than iron, but lower than magnesium. Top guy wins.
- 3Zn +FeCl3 >> Fe +3 ZnCl this works, the zinc replaces the iron in the iron(III)chloride solution, and the iron becomes a solid metal
- but this will not happen: Zn + MgCl2 >NOT> Mg + ZnCl The zinc metal stays zinc metal. No reaction takes place.
Understanding this is not that important at this point, but I know some of you like to be precise just so you know that some reactions are likely to happen, and some are not.
Do these worksheets:
http://www.sciencegeek.net/Chemistry/chempdfs/EquationsWorksheet4.pdf
and http://www.sciencegeek.net/Chemistry/chempdfs/EquationsWorksheet5.pdf
[some of these reactions also won't really happen, as you will see from the answer sheet;
don't worry about trying to predict this with the solubility table, just do the switcharoo >>
AB + CD -> AD + CB and make sure your compounds are metalfirst,thennonmetal.
........................................
Hope you are enjoying the labwork, and putting it together with the worksheets, and starting to "get it."
I appreciate your feedback; we are in the home stretch of chemistry; keep up the good work,
Thursday, March 5, 2015
For more pictures of St. Basil's Cathedral, use Google image or go here:
My own google search of St. Basil's
Feel free to use any of these pictures for inspiration,
or find your own landmark. I suggest you print out about 3 different views to assist you,
and to doodle around with it through the week.



...and back to science, good job today doing the Synthesis of copper(II)oxide.
Monday we will do more experiments, probably on Decomposition reactions.
1. Answer the questions on the experiment page from class (in your SJ) be thorough.
2.Video - take notes in your SJ
3. Worksheet: Decomposition Reactions print page 1 only and check your answers from page 2
My own google search of St. Basil's
Feel free to use any of these pictures for inspiration,
or find your own landmark. I suggest you print out about 3 different views to assist you,
and to doodle around with it through the week.



...and back to science, good job today doing the Synthesis of copper(II)oxide.
Monday we will do more experiments, probably on Decomposition reactions.
1. Answer the questions on the experiment page from class (in your SJ) be thorough.
2.Video - take notes in your SJ
3. Worksheet: Decomposition Reactions print page 1 only and check your answers from page 2
Monday, March 2, 2015
Types of Chemical Reactions
Today's class did not go so well...I think we will move on...
to>>>CHEMICAL REACTIONS!!! ---the Big Five
- synthesis (aka composition) -- putting together
- decomposition -- taking apart
- single displacement -- a switcharoo
- double displacement --a double -switcharoo
- combustion -- and kapow!
An overview in videos: yes, take notes!
This information is also in your textbook, for reference, on pages 746-749, which you should read and answer the questions in the math skills activity and in the assessment.
and here is a worksheet:
answers are on the second page
On Thursday, we will do a lab involving a synthesis reaction between copper and oxygen. Please be on time.
And here are some interesting videos on HCl...hydrochloric acid,,,
which you will remember we mixed with zinc in class, producing hydrogen gas...
DON'T FORGET TO COME PREPARED FOR ART ON THURSDAY
SEE LAST WEEK'S BLOG
Thursday, February 26, 2015
Art AND Science Homework
Art Students: Prepare for the Big One! So far we have done small watercolor projects, in preparation...now a major assignment begins, combining what you are exploring in your geography class and your artistic genius. An 11x15 watercolor, and it can be...



- a famous landmark/building from a country you've studied, or are interested in, like the Hagia Sophia, or the Taj Mahal or ...anything except the USA
- an artistically done map which reflects the culture of the area as well as the geography
- or your idea, so long as it reflects a geographical theme, and it gets my approval
TO PREPARE>>> Collect several pictures (Google image search) and study up on what you are proposing to create. Bring to Thursday's class for approval. This project will require you to do some work at home and come to class prepared.



AND NOW FOR SCIENCE>>>>>>>>>>>>>>>>>>>>>>>>
This worksheet: http://misterguch.brinkster.net/PRA001.pdf
and also this worksheet >>>https://drive.google.com/file/d/0B07s3-bcUr7teWQ2UjVIckI0bFU/view?usp=sharing
Turn in all worksheets on Monday
Monday, February 23, 2015
Ketchup...I mean, Catch Up
If you have not done the homework from last blog, please scroll down to find last week's blog and put in the extra time. Three worksheets and two videos....this is catching up, and you have till next Monday. You also have the review worksheet from class to complete.
****But to be clear, for this Thursday, you MUST watch the following videos, and please take notes.
***** Thursday's lab will be on counting atoms.
****But to be clear, for this Thursday, you MUST watch the following videos, and please take notes.
***** Thursday's lab will be on counting atoms.
*****Don't forget to finish up the worksheet from class.
Friday, February 13, 2015
Over the Break...
No class next week, but there is homework for Monday when we return.
And here is an excellent video, please watch the first 14 minutes at least, but you may certainly finish it off, to get ahead. Quantifying Chemical Reactions not a catchy name, but really well done and interesting. total time 28 minutes.
LOOKING AHEAD; When we come back from winter break, we will dig into the subject of chemical reactions-- lotsa labs, lotsa fun. We will once again do individual demonstrations of chemical reactions and the internet is a great source to find interesting demonstrations using household products. So keep your eyes open for an experiment you might want to demonstrate to the class.
PRINT OUT THESE THREE WORKSHEETS
answers are included, but save ink and only print the first page of each file.
- Mixed ionic and covalent naming worksheet
- Word equations worksheet (just write the balanced equation; you can ignore the "H=negative" stuff ) if you can figure out if it is dissolved in water (aq), solid (s), liquid (l) or gas (g) write that as a subscript by the compound. If heat is a product, write it on the product side as "+heat"
- More balancing equations worksheet
And here is an excellent video, please watch the first 14 minutes at least, but you may certainly finish it off, to get ahead. Quantifying Chemical Reactions not a catchy name, but really well done and interesting. total time 28 minutes.
LOOKING AHEAD; When we come back from winter break, we will dig into the subject of chemical reactions-- lotsa labs, lotsa fun. We will once again do individual demonstrations of chemical reactions and the internet is a great source to find interesting demonstrations using household products. So keep your eyes open for an experiment you might want to demonstrate to the class.
Monday, February 9, 2015
Like Sudoku, but different...
I. pHET simulation : Balancing Chemical Equations - Play the Game! do the introduction, use the tools (in the upper right corner) play the game (3 levels) -- I can't tell if it is easier to start with the sim or with the video... if the sim is frustrating, come back to it after the lesson.
...and on to the [skip,skip,skip] the next chapter, Chapter 24, Chemical Reactions!
Read section 1, Chemical Changes, page 738-742
Take notes in your SJ, including vocabulary, reading check questions, copy Table 1 and Figure 4 and answer the section 1 assessment questions 1-7.
II. Balancing Chemical Equations
Now do the Balancing equations worksheet from class:
for help with harder equations, watch more:
And worksheet/answers are here:http://misterguch.brinkster.net/WKS001_019_348432.pdf
not to belabor...
...and on to the [skip,skip,skip] the next chapter, Chapter 24, Chemical Reactions!
Read section 1, Chemical Changes, page 738-742
Take notes in your SJ, including vocabulary, reading check questions, copy Table 1 and Figure 4 and answer the section 1 assessment questions 1-7.
II. Balancing Chemical Equations
Now do the Balancing equations worksheet from class:
for help with harder equations, watch more:
Friday, February 6, 2015
X-planations
Explain It: As I said in class, go over your lab packet, add any additional information you need, if you skipped a lab, google it on youtube, and then for each lab make a very short statement of explanation---which principle is demonstrated?-- using the vocabulary from last blog.
Reading: Ask me a chemistry question: the pepper-soap experiment
Videos:
Print out page 2 only, do set 3 only. Correct your own work from page 4.
chemical formula writing worksheet Don't forget the cross-over method for balancing charges, and it may be helpful to write the chemical symbol and charge in the cation column.
Monday, February 2, 2015
The Chemistry of Water
Some Videos on the Chemistry of Water -- repetitive, but all the better to remember, my dearies...
Take notes/illustrate. If the information is repeated, you only have to write it down once.
Some vocabulary to watch for:
Polar Covalent...Adhesion...Cohesion...Capillary Action...hydrophilic & hydrophobic... Universal Solvent... Heat Capacity
++++++++++++++++++++++++++++++++++++++++++++++++++++
Worksheet Time :)
This will make your hand sore...
Worksheet: Naming Ionic Compounds containing Polyatomic Ions
Answer sheet
And now, a word from our sponsors...Water Is Life...
Take notes/illustrate. If the information is repeated, you only have to write it down once.
Some vocabulary to watch for:
Polar Covalent...Adhesion...Cohesion...Capillary Action...hydrophilic & hydrophobic... Universal Solvent... Heat Capacity
++++++++++++++++++++++++++++++++++++++++++++++++++++
Worksheet Time :)
This will make your hand sore...
Worksheet: Naming Ionic Compounds containing Polyatomic Ions
Answer sheet
And now, a word from our sponsors...Water Is Life...
Friday, January 30, 2015
A few comments about our two electrolysis experiments:
Here's what I want you to understand-->>>
First of all, doing the experiment gives you hands-on experience in observing chemical reactions. It is important to follow directions carefully, including what's in the diagram, so that you get your setup just right. Then it is important to be patient, not everything is an explosion, or has an immediately visible reaction. Sometimes no reaction is what is happening, and sometimes a slow reaction is what you have to observe --patience and attention to detail!
- In these experiments, the main idea is that transferring electrons changes the chemical and physical properties of that atom, DRAMATICALLY!
- In the first lab, the chloride ion [Cl-] lost an electron, combined with another Cl-, and bubbled up as chlorine gas. Likewise, the H+ atoms gained an electron, combined to form H2 and bubbled up as a gas.
- In yesterday's demonstration, the copper (II) ion, which was dissolved in the blue solution, gained two electrons and became the regular metallic copper that we are used to seeing on a penny. We did not observe it, but the sulfate ion [SO42- ] lost two electrons, combined with hydrogen, and became sulfuric acid.
- It took energy (from the battery) to make these reactions happen.
- A chemical reactions is when compounds break apart and recombine to form different combinations of atoms.
- I also wanted you to see examples of chemical equations look like, just to get used to them:
- 2H+ + 2e-
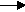 H2 (hydrogen gas forms at the (-)cathode).
H2 (hydrogen gas forms at the (-)cathode).
2Cl- - 2e-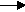 Cl2 (chlorine gas forms at the (+)anode).
Cl2 (chlorine gas forms at the (+)anode). - 2NaCl(aq) + 2H2O(l)
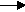 2Na+(aq) + 2OH-(aq) + Cl2(g)+ H2(g)
2Na+(aq) + 2OH-(aq) + Cl2(g)+ H2(g) - Matter is not created or destroyed-- The Law of the Conservation of Matter-- means that if we consider matter at the atomic level, the small particles are not created or destroyed. We can trace these atoms and molecules through a system. The same atoms present at the beginning are there at the end, just in different combinations.
NOMENCLATURE FOR COVALENT BONDING
Worksheet # 3 from this
http://www.npsd.k12.nj.us/cms/lib04/NJ01001216/Centricity/Domain/474/Ch%209%20WS%20Ionic%20Tranisition%20Covalent%20Nomenclature%20Packet.pdf
and the answers:
For INSTRUCTIONS, SEE YOUR TEXTBOOK, p. 592-593 and/or watch this:
Memorize the PREFIXES
optional, but interesting, no notes necessary.
Monday, January 26, 2015
ELECTROLYSIS!!!!!!!!!!!!!!!!
Fun class today; thank you. I'm guessing, however, that there was not enough time to write down your observations, so let's start with that, for homework. Just jot down notes (be descriptive) on the original directions from class, next to figure 2, and answer the questions that I have highlighted
here's some videos:
actually, that "POP' was just hydrogen
brine is actually salt water
Now for some reason, I can't upload this from youtube, so go here:
Draw this diagram in your science journal.

Print this: Nomenclature Packet Print pages 1-2 [skip 3rd page]
PAGE ONE- review (like the last worksheet) PAGE TWO - like what we did in class with the metals that can have more than one kind of charge. See textbook section 3 for a reminder, or watch this video: https://www.youtube.com/watch?v=_w6-4fRQt1Y. Here is the periodic table with oxidation numbers to help: Periodic table of IONS You should print this out and put it on the other side of your binder cover, or somewhere you can easily find it.
nomenclature - ANSWERS
last note, was that you, Jillian, who said there were mistakes on last class' answer sheet? Right you are! We'll review this stuff on Thursday.
Thursday, January 22, 2015
Binomial Nomenclature!
This was once Lily's favorite word. :) Along with "geofluvial morphology" -- thank you Mrs. Ripple!
First of all, REVIEW chapter so far...and read the first part of section 3, pages 587-590. Highlight the important bits, and work the problems in the blue boxes. There's a worksheet from your packet of textbook worksheets on section 3 reinforcement (something about the cross-over method?) that you should do,
Writing Ionic Formulas: The Movie
And then do the worksheet from class! here's the worksheet, in case you missed it
Answers here: Easy-Peasy 123z scroll down to page 2. Mrs Grammar Nazi wants you to notice that YOU DO NOT CAPITALIZE the names of ionic compounds, unless it begins the sentence.
Lastly, Movie II - What Is a Polyatomic Ion?
Coming Soon - Electrolysis!
Breaking Bonds
Have a Wonderful Weekend!
Monday, January 19, 2015
Drawing the Lewis Dot Structure of a Molecule...
First, a review of bonding...
And now the Lewis Structures of a Molecule...Watch this video and take notes on the five steps.
Following these 5 steps, work through the handout from class, starting with F and proceeding to the next page, and doing the first two rows on the backside. Anything else is extra credit. If you are stuck, type in "Lewis dots for co2", for example, in the search box at the top of the youtube page, and Dr. B will walk you through the steps. [****ELECTRONEGATIVITY: the ability to attract electrons to itself***]
Check your answers here: front side back side
Keep your science notebpook up to date, and your binder organized! Don't throw away handouts; review all your chapter notes/highlights each homework period. This will only take a few minutes and will pay off HUGHLY.
And now the Lewis Structures of a Molecule...Watch this video and take notes on the five steps.
Following these 5 steps, work through the handout from class, starting with F and proceeding to the next page, and doing the first two rows on the backside. Anything else is extra credit. If you are stuck, type in "Lewis dots for co2", for example, in the search box at the top of the youtube page, and Dr. B will walk you through the steps. [****ELECTRONEGATIVITY: the ability to attract electrons to itself***]
Check your answers here: front side back side
Keep your science notebpook up to date, and your binder organized! Don't throw away handouts; review all your chapter notes/highlights each homework period. This will only take a few minutes and will pay off HUGHLY.
Friday, January 16, 2015
Bonding Time
bonding videos: take notes in your science journals!
Last video: Khan Academy 13 minutes
Take notes.
Now read your text book (again): Chapter 19, section 2 Types of Bonds and highlight the parts you understand are important to remember for a test. Pictures, too.
worksheet page 30, (from worksheet packet)
and if you have not yet done so, pages 20, 27, 28
Those of you who did not do the Gizmos from last blog, do those this weekend! It is just as important to find a way to do the homework, as it is to do the homework.
Subscribe to:
Posts (Atom)
